Ipertrofia prostatica benigna
Pubblicato il: 29/06/2017
| Ultimo aggiornamento: 21/11/2023
L’ipertrofia prostatica benigna è nota anche con l’acronimo italiano IPB (Iperplasia prostatica benigna) o quello inglese BPH (benign prostatic Hiperplasia). In maniera inopportuna viene definita anche adenoma della prostata BEP (benign elargement prostate). Si tratta di una tra le patologie benigne più comuni nell’uomo.
Questa condizione patologica si contraddistingue per un aumento di volume della ghiandola prostatica.
Il suo sviluppo è legato ad un fisiologico invecchiamento dell’organismo, nonché da una lenta e graduale trasformazione dell’assetto ormonale del sistema endocrino sessuale.
L’IPB presenta una bassa incidenza negli uomini con età inferiore ai 40 anni. In questo caso, la percentuale si attesta intorno all’8%. Aumenta, invece, in maniera progressiva con il trascorrere degli anni. Negli uomini over 60, infatti, si attesta in percentuali superiori al 50.
Il tasso di mortalità relativo all’Ipertrofia prostatica benigna è basso: si parla, infatti, di uno 0,35 su 100.000. Va sottolineato, comunque, che, se non monitorata, può far evidenziare un aggravamento progressivo, con un conseguente peggioramento dello stato di benessere del paziente.
Questa condizione patologica si contraddistingue per un aumento di volume della ghiandola prostatica.
Il suo sviluppo è legato ad un fisiologico invecchiamento dell’organismo, nonché da una lenta e graduale trasformazione dell’assetto ormonale del sistema endocrino sessuale.
L’IPB presenta una bassa incidenza negli uomini con età inferiore ai 40 anni. In questo caso, la percentuale si attesta intorno all’8%. Aumenta, invece, in maniera progressiva con il trascorrere degli anni. Negli uomini over 60, infatti, si attesta in percentuali superiori al 50.
Il tasso di mortalità relativo all’Ipertrofia prostatica benigna è basso: si parla, infatti, di uno 0,35 su 100.000. Va sottolineato, comunque, che, se non monitorata, può far evidenziare un aggravamento progressivo, con un conseguente peggioramento dello stato di benessere del paziente.
Approfondimenti
 Cause
Cause
La prostata, conosciuta anche come ghiandola prostatica, è una ghiandola che appartiene dell'apparato genitale maschile. Il suo compito principale è quello di produrre ed emettere il liquido prostatico, una delle componenti dello sperma, che contiene gli elementi utili per nutrire e veicolare gli spermatozoi.Nel corso degli anni, il processo di invecchiamento provoca un calo relativo della produzione di testosterone. Ciò comporta, di conseguenza, una maggiore attività della componente estrogenica endogena, che determina un aumento dello stimolo della crescita della componente centrale fibro-muscolare della prostata da cui genera l’IPB.
In virtù della sua localizzazione anatomica, la ghiandola riveste, dunque, un ruolo strategico nel processo di svuotamento delle vie urinarie. Questo perché, mediante la prostata, passa l’uretra, ovvero il canale attraverso cui decorrono le urine ed il liquido seminale.
Si intuisce facilmente che, qualsivoglia processo evolutivo, benigno o maligno che sia, ostacola la zona centrale della prostata. Tale processo, inoltre, è responsabile di malattie che interessano le vie escretrici, in particolare la vescica.
Il ruolo evolutivo dell’Ipertrofia prostatica benigna è decisivo sui sintomi delle vie urinarie (LUTS), così come anche sul benessere di un individuo.
 Sintomi
Sintomi
Il graduale aumento di volume della porzione centrale della prostata, responsabile della genesi dell’ipertrofia prostatica benigna, comporta un restringimento lento e progressivo dell’uretra prostatica (si tratta della parte della ghiandola che comincia con l'orifizio uretrale interno della vescica, per terminare all'apice del pene, corrispondentemente all'orifizio uretrale esterno).Il primo elemento sintomatologico, generalmente ravvisabile è, quasi sempre, la diminuzione del calibro e del getto urinario. Di frequente, si associa anche a difficoltà nel cominciare la minzione.
La sintomatologia, poi, potrebbe essere completata da altri segni, quali:
- bisogno di urinare più frequentemente del normale;
- il bisogno di urinare di notte, ovvero la nicturia;
- la necessità di urinare, talvolta con perdita involontaria di qualche goccia di urina. In tal caso si parla di urgenza minzionale;
- una minzione a più tempi, definita intermittente;
- la sensazione di non aver svuotato completamente la vescica;
- difficoltà a cominciare la minzione, sebbene la presenza di un forte stimolo;
- dopo aver urinato, il paziente nota la fuoriuscita di alcune gocce di urina. Si parla, in tal caso, di gocciolamento post-minzionale.
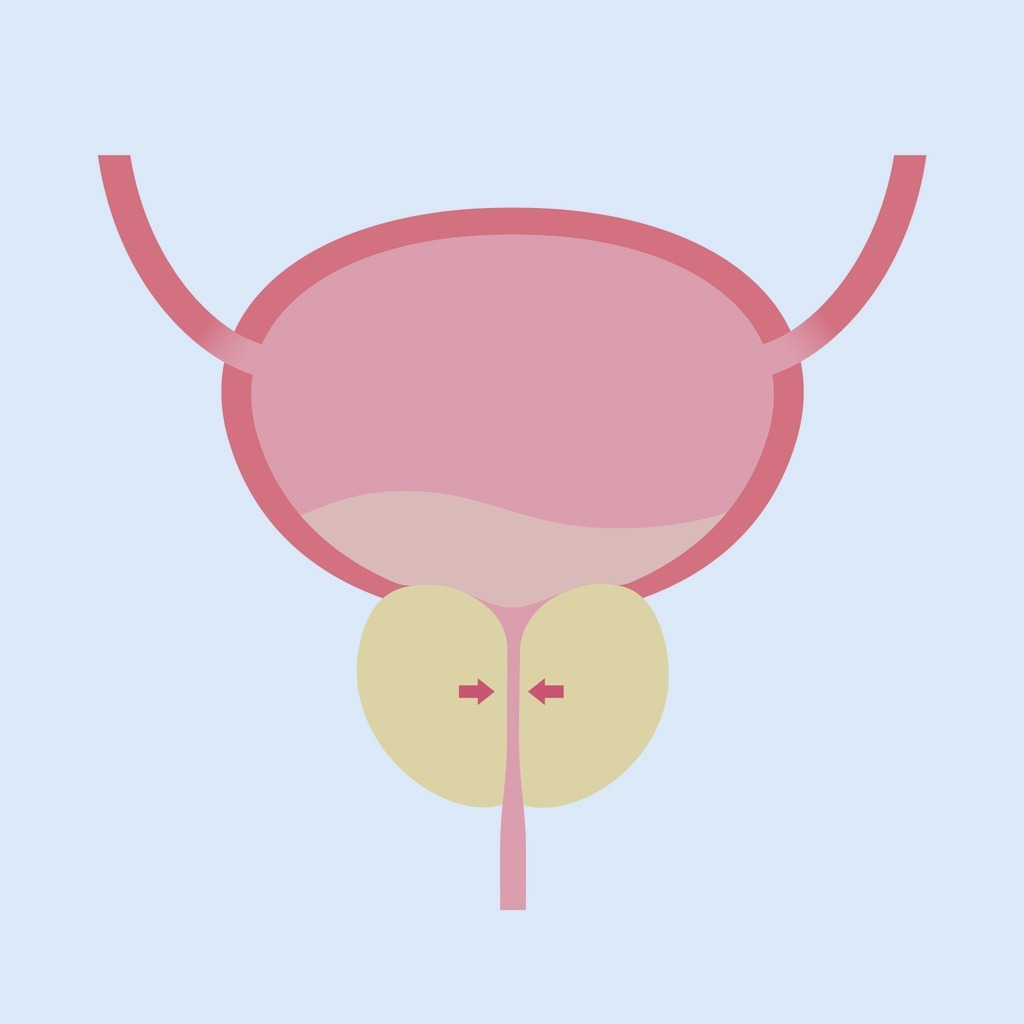
 Diagnosi
Diagnosi
Per eseguire una diagnosi di ipertrofia prostatica, attualmente i cardini sono rappresentati da tre step in particolare, ovvero:- un’anamnesi scrupolosa. Si tratta di un aspetto importante, perché può mostrare patologie parallele (endocrinologiche, dismetaboliche e cardiovascolari);
- l’IPSS (International Prostate Symptom Score), ovvero un questionario valutativo, che, mediante un punteggio, analizza l’importanza dei sintomi urinari sulla qualità di vita;
- l’esame obiettivo, che prevede l’esplorazione rettale, nota anche come DRE digital rectal eploration.
- esame urine e urinocoltura con ev ABG;
- esami ematochimici della funzionalità renale (azotemia, creatinina, acido urico, elettroliti, emocromo completo);
- l’uroflussimetria e, eventualmente, l’esame urodinamico;
- al tempo stesso, nell'ambito della visita, allo scopo di ottenere una corretta valutazione clinica, viene richiesto l’esame ematochimico del PSA (antigene prostatico specifico). I valori di tale esame devono essere interpretati, in modo da valutare la coesistenza di una neoplasia prostatica;
- l’ecografia prostatica transrettale, che ha l’obiettivo di valutare il volume prostatico, qualora l’esplorazione rettale abbia dato esiti sospetti, e, soprattutto, ai fini di un eventuale intervento chirurgico.
 Rischi
Rischi
Una possibile evoluzione dell'ipertrofia prostatica benigna, laddove non trattata, il più delle volte, può comportare delle complicazioni. Si tratta di complicanze correlate a uno svuotamento incompleto della vescica, nonché al ristagno urinario in vescica (ovvero la ritenzione urinaria). Tra le complicazioni più comuni, possiamo elencare:- infezioni urinarie ricorrenti;
- ematurie (ovvero presenza di globuli rossi nelle urine) frequenti e recidivanti;
- litiasi vescicale o comunemente note come calcolosi vescicale. Questa condizione patologica vescicale è legata ad una ritenzione urinaria reiterata nel tempo. Essa provoca un addensamento urinario di minerali urinari nel fondo vescicale, che è all’origine della formazione dei calcoli. Questa condizione comporta sanguinamento (ematuria), dolore e infezioni urinarie;
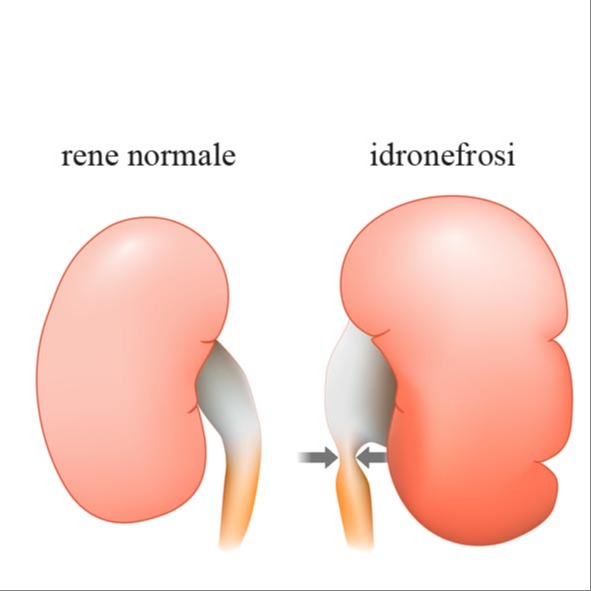
- insufficienza renale cronica, data da una ritenzione urinaria cronica che, provocando un aumento della pressione idrostatica intra-vescicale, determina una dilatazione delle vie urinarie alte (idronefrosi), con compromissione irreversibile della filtrazione dell'emuntorio renale;
- compromissione della funzionalità vescicale, secondaria ad un eccesivo iper-lavoro della muscolatura detrusoriale. Questa situazione è legata all'ostruzione dell'adenoma prostatico, a livello dell'uretra prostatica, comportando una pressione idrostatica endovescicale che cresce in maniera progressiva, definendo una lenta e graduale perdita di forza contrattile ed elastica. Ne consegue ipocontrattilità e ritenzione urinaria.
 Cure e Trattamenti
Cure e Trattamenti
Non tutti i soggetti affetti da ipertrofia prostatica benigna devono obbligatoriamente essere sottoposti a una specifica terapia, poiché possono rientrare in un protocollo definito di vigile sorveglianza. Ovviamente, questo è possibile solo in seguito a un monitoraggio clinico e strumentale.Per quanto riguarda la terapia medica, questa annovera:
- farmaci chimici o di sintesi (Inibitori delle 5 alfa reduttasi, Alfa-litici), che comprendono le seguenti molecole: Finasteride e Dutasteride. Tali farmaci presentano un meccanismo d’azione similare e sono i soli capaci di bloccare l’evoluzione dell’IPB;
- gli alfa litici sono la classe di farmaci di sintesi. Si tratta di medicinali particolarmente utilizzati per il trattamento dell’ipertrofia prostatica benigna e comprendono le seguenti molecole: AlfuzosinaI, Tamsulosina, terazosina; Silodosina. Il loro processo di azione si realizza mediante il rilassamento della muscolatura liscia del collo vescicale.
Ci sono poi i farmaci naturali, conosciuti anche come fitofarmaci. Si tratta di medicinali che hanno una derivazione vegetale. Tra questi, quelli maggiormente utilizzati sono la serenoa repens, il pygeum africanum, l’ortica dioica e i semi di zucca. Tali sostanze operano in maniera analoga ai farmaci chimici. La loro modalità di azione include:
- inibizione delle 5 alfa reduttasi;
- rilassamento del collo vescicale;
- azione antiproliferativa sulle cellule prostatiche.
Altri interventi, invece, possono essere di chirurgia endoscopica o di endo-urologia, tra i quali è possibile annoverare:
- incisione trans-uretrale della prostata (TUIP);
- resezione trans-uretrale della prostata (TURP);
- vaporizzazione laser della prostata (PVP);
- prostatectomia trans-uretrale mediante laser al tullio (TULEP);
- enucleazione laser olmio adenoma della prostata (HOLEP).
N.B.: L’articolo in questione, scritto in maniera semplice ed essenziale, si pone l’obiettivo di fornire elementi di conoscenza, che possono essere utili al fine di essere d’aiuto ad un lettore o paziente, nel comprendere e/o rendersi edotto su una delle patologie benigne più comuni nell’uomo adulto.
Bibliografia
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplas J Urol 2011 May;185(5):1793- 803. PMID: 21420124.
- Jarvis TR, Chughtai B, Kaplan SA. Bladder Outlet Obstruction and BPH. Current Bladder Dysfunction Reports 2014:1-7.
- Abrams P, Chapple C, Khoury S, et al. Evaluation and treatment of lower urinary tract symptoms in older men. J Urol 2013 Jan;189(1 Suppl):S93-S101. PMID: 23234640.
- McVary K, Roehrborn C, Avins A. American Urological Association Guideline: Management of Benign Prostatic Hyperplasia (BPH) Revised, 2010. American Urological Association Education and Research: Inc; 2010.
- Suarez O, Osborn D, Kaufman M, et al. Mirabegron for male lower urinary tract symptoms. Current urology reports 2013;14(6):580-4. PMID: 23913200.
- Kaplan SA. Re: beta3-adrenoreceptor agonist mirabegron is effective for overactive bladder that is unresponsive to antimuscarinic treatment or is related to benign prostatic hyperplasia in men. J Urol 2014 May;191(5):1344-6. PMID: 24745510.
- Micromedex 2.0. Truven Health Analytics: Micromedex Solutions. Greenwood Village, CO: Truven Health Analytics, Inc; 2015.
- Viswanathan M, Ansari M, Berkman N, et al. Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions: AHRQ. 2012.
- Fu R, Gartlehner G, Grant M, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. Journal of Clinical Epidemiology 2011 Nov;64(11):1187-97. PMID: 21477993.
- IntHout J, Ioannidis JP, Borm GF. The Hartung- Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC medical research methodology 2014;14(1):25. PMID: 24548571.
- StataCorp. Stata Statistical Software: Release 14 In: LP. S, ed. College Station, TX; 2015.
- The Cochrane Collaboration. Review Manager (RevMan) [Computer program]. Version 5.2. 2012.
- Higgins J, Green S. Cochrance Handbook for Systematic Reviews of Interventions Version 5.1. 0. The Cochrane Collaboration, 2011 [2013.5. 16]; 2011.
- Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol 1995 Nov;154(5):1770-4. PMID: 7563343.
- Johnston BC, Patrick DL, Busse JW, et al. Patient- reported outcomes in meta-analyses--Part 1: assessing risk of bias and combining outcomes. Health Qual Life Outcomes 2013;11:109. PMID: 23815754.
- Berkman ND, Lohr KN, Ansari M, et al. Grading the strength of a body of evidence when assessing health care interventions for the effective health care program of the Agency for Healthcare Research and Quality: an update. 2013. PMID: 24404627.
- Atkins D, Chang S, Gartlehner G, et al. Assessing the Applicability of Studies When Comparing Medical Interventions. 2010 December. Available at: http://www.effectivehealthcare.ahrq.gov/index.cfm/se arch-for-guides-reviews-and- reports/?pageaction=displayProduct&productID=603# 2412. Accessed AHRQ Publication No. 11-EHC019- EF.
- Seki N, Takahashi R, Yamaguchi A, et al. Non- inferiority of silodosin 4mg once daily to twice daily for storage symptoms score evaluated by the International Prostate Symptom Score in Japanese patients with benign prostatic hyperplasia: A multicenter, randomized, parallel-group study. Int J Urol 2015 01 Mar;22(3):311-6. PMID: 25597862.
- Novara G, Chapple CR, Montorsi F. Individual patient data from registrational trials of silodosin in the treatment of non-neurogenic male lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH): subgroup analyses of efficacy and safety data. BJU Int. 2015 May;115(5):802-14. PMID: 25130493.
- Pande S, Hazra A, Kundu AK. Evaluation of silodosin in comparison to tamsulosin in benign prostatic hyperplasia: a randomized controlled trial. Indian J Pharmacol 2014 Nov-Dec;46(6):601-7. PMID: 25538330.
- Choo MS, Song M, Kim JH, et al. Safety and efficacy of 8-mg once-daily vs 4-mg twice-daily silodosin in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia (SILVER Study): a 12- week, double-blind, randomized, parallel, multicenter study. Urology 2014 Apr;83(4):875-81. PMID: 24529580.
- Yokoyama T, Hara R, Fujii T, et al. Comparison of Two Different alpha1-Adrenoceptor Antagonists, Tamsulosin and Silodosin, in the Treatment of Male Lower Urinary Tract Symptoms Suggestive of Benign Prostatic Hyperplasia: A Prospective Randomized Crossover Study. LUTS: Lower Urinary Tract Symptoms 2012 January;4(1):14-8. PMID: - 26676453.
- Yu HJ, Lin AT, Yang SS, et al. Non-inferiority of silodosin to tamsulosin in treating patients with lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH). BJU Int 2011 Dec;108(11):1843-8. PMID: 21592295.
- Yokoyama T, Hara R, Fukumoto K, et al. Effects of three types of alpha-1 adrenoceptor blocker on lower urinary tract symptoms and sexual function in males with benign prostatic hyperplasia. Int J Urol 2011 Mar;18(3):225-30. PMID: 21272091.
- Watanabe T, Ozono S, Kageyama S. A randomized crossover study comparing patient preference for tamsulosin and silodosin in patients with lower urinary tract symptoms associated with benign prostatic hyperplasia. Journal of International Medical Research 2011;39(1):129-42. PMID: 21672315.
- Chapple CR, Montorsi F, Tammela TL, et al. Silodosin therapy for lower urinary tract symptoms in men with suspected benign prostatic hyperplasia: results of an international, randomized, double-blind, placebo- and active-controlled clinical trial performed in Europe. Eur Urol 2011 Mar;59(3):342-52. PMID: 21109344.
- Miyakita H, Yokoyama E, Onodera Y, et al. Short- term effects of crossover treatment with silodosin and tamsulosin hydrochloride for lower urinary tract symptoms associated with benign prostatic hyperplasia. Int J Urol 2010 Oct;17(10):869-75. PMID: 20735791.
- Marks LS, Gittelman MC, Hill LA, et al. Rapid efficacy of the highly selective alpha1A-adrenoceptor antagonist silodosin in men with signs and symptoms of benign prostatic hyperplasia: pooled results of 2 phase 3 studies. J Urol 2009 Jun;181(6):2634-40. PMID: 19371887.
- Kawabe K, Yoshida M, Homma Y, et al. Silodosin, a new alpha1A-adrenoceptor-selective antagonist for treating benign prostatic hyperplasia: results of a phase III randomized, placebo-controlled, double- blind study in Japanese men. BJU Int 2006 Nov;98(5):1019-24. PMID: 16945121.
- Novara G, Chapple CR, Montorsi F. A pooled analysis of individual patient data from registrational trials of silodosin in the treatment of non-neurogenic male lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH). BJU Int 2014 Sep;114(3):427-33. PMID: 24571313.
- Eisenhardt A, Schneider T, Cruz F, et al. Consistent and significant improvement of nighttime voiding frequency (nocturia) with silodosin in men with LUTS suggestive of BPH: pooled analysis of three randomized, placebo-controlled, double-blind phase III studies. World J Urol 2014 Oct;32(5):1119-25. PMID: 24442560.
- Marks LS, Gittelman MC, Hill LA, et al. Rapid efficacy of the highly selective alpha(1A)- adrenoceptor antagonist silodosin in men with signs and symptoms of benign prostatic hyperplasia: pooled results of 2 phase 3 studies. J Urol 2013 Jan;189(1 Suppl):S122-8. PMID: 23234617.
- Roehrborn CG, Kaplan SA, Lepor H, et al. Symptomatic and urodynamic responses in patients with reduced or no seminal emission during silodosin treatment for LUTS and BPH. Prostate Cancer and Prostatic Diseases 2011 June;14(2):143-8. PMID: 21135869.
- Kaplan SA, Roehrborn CG, Hill LA, et al. Effect of estimated prostate volume on silodosin-mediated improvements in the signs and symptoms of BPH: does prostate size matter? Open Access Journal of Urology 2011;3:89-93. PMID: 24198640.
- Gittelman MC, Marks LS, Hill LA, et al. Effect of silodosin on specific urinary symptoms associated with benign prostatic hyperplasia: Analysis of international prostate symptom scores in 2 phase III clinical studies. Open Access Journal of Urology 2011 (3 ):1-5. PMID:24198629.
- Homma Y, Kawabe K, Takeda M, et al. Ejaculation disorder is associated with increased efficacy of silodosin for benign prostatic hyperplasia. Urology 2010 Dec;76(6):1446-50. PMID: 20472263.
- Singh I, Garg G, Agarwal V. 'Tamsulosin and darifenacin versus 'tamsulosin monotherapy' for 'BPH with accompanying overactive bladder'. Journal of Clinical and Diagnostic Research 2015 01 Jun;9(6):PC08-PC11. PMID: 26266159.
- Liao CH, Kuo HC. How to choose first-line treatment for men with predominant storage lower urinary tract symptoms: a prospective randomised comparative study. Int J Clin Pract 2015 Jan;69(1):124-30. PMID: 25495719.
- Memon I, Javed A, Pirzada AJ, et al. Efficacy of Alfuzosin with or without Tolterodine, in Benign Prostatic Hyperplasia (BPH) having irritative (overactive bladder) symptoms. Rawal Medical Journal 2014;39(4):421-4. PMID: 2014928955.
- Lee SH, Byun SS, Lee SJ, et al. Effects of initial combined tamsulosin and solifenacin therapy for overactive bladder and bladder outlet obstruction secondary to benign prostatic hyperplasia: a prospective, randomized, multicenter study. International urology and nephrology 2014;46(3):523- 9. PMID: 24097273.
- Ko K, Yang DY, Lee WK, et al. Effect of improvement in lower urinary tract symptoms on sexual function in men: Tamsulosin monotherapy vs. combination therapy of tamsulosin and solifenacin. Korean Journal of Urology 2014;55(9). PMID: 25237463.
- Van Kerrebroeck P, Haab F, Angulo JC, et al. Efficacy and safety of solifenacin plus tamsulosin OCAS in men with voiding and storage lower urinary tract symptoms: results from a phase 2, dose-finding study (SATURN). Eur Urol 2013 Sep;64(3):398-407. PMID: 23537687.
- Van Kerrebroeck P, Chapple C, Drogendijk T, et al. Combination therapy with solifenacin and tamsulosin oral controlled absorption system in a single tablet for lower urinary tract symptoms in men: efficacy and safety results from the randomised controlled NEPTUNE trial. Eur Urol 2013 Dec;64(6):1003-12. PMID: 23932438.
- Konstantinidis C, Samarinas M, Andreadakis S, et al. Lower urinary tract symptoms associated with benign prostatic hyperplasia: combined treatment with fesoterodine fumarate extended-release and tamsulosin--a prospective study. Urol Int 2013;90(2):156-60. PMID: 23221480.
- Kaplan SA, He W, Koltun WD, et al. Solifenacin plus tamsulosin combination treatment in men with lower urinary tract symptoms and bladder outlet obstruction: a randomized controlled trial. Eur Urol 2013;63(1):158-65. PMID 22831853.
- Malkoc E, Ates F, Senkul T, et al. Additive role of trospium chloride in the management of men with voiding and storage symptoms. Turkiye Klinikleri Journal of Medical Sciences 2012;32(5):1374-80.
- Ceylan C, Ertas K, Dogan S, et al. The Comparison alpha-blocker+M3 selective anti-muscarinic combined therapy and alpha-blocker monotherapy. Journal of Clinical and Analytical Medicine 2012 January;3(1):33-5.
- Yamaguchi O, Kakizaki H, Homma Y, et al. Solifenacin as add-on therapy for overactive bladder symptoms in men treated for lower urinary tract symptoms--ASSIST, randomized controlled study. Urology 2011 Jul;78(1):126-33. PMID: 21601248.
- Seo DH, Kam SC, Hyun JS. Impact of lower urinary tract symptoms/benign prostatic hyperplasia treatment with tamsulosin and solifenacin combination therapy on erectile function. Korean Journal of Urology 2011 January;52(1):49-54. PMID:21344031.
- Lee SH, Chung BH, Kim SJ, et al. Initial combined treatment with anticholinergics and alpha-blockers for men with lower urinary tract symptoms related to BPH and overactive bladder: a prospective, randomized, multi-center, double-blind, placebo-controlled study. Prostate Cancer Prostatic Dis 2011 Dec;14(4):320-5. PMID: 21788967.
- Kaplan SA, Roehrborn CG, Gong J, et al. Add-on fesoterodine for residual storage symptoms suggestive of overactive bladder in men receiving alpha-blocker treatment for lower urinary tract symptoms. BJU Int 2011 Jun;109(12):1831-40. PMID: 21966995.
- Chung SD, Chang HC, Chiu B, et al. The efficacy of additive tolterodine extended release for 1-year in older men with storage symptoms and clinical benign proastatic hyperplasia. Neurourol Urodyn 2011 Apr;30(4):568-71. PMID: 21344494.
- Kaplan SA, McCammon K, Fincher R, et al. Safety and tolerability of solifenacin add-on therapy to alpha- blocker treated men with residual urgency and frequency. J Urol 2009 Dec;182(6):2825-30. PMID: 19837435.
- Chapple C, Herschorn S, Abrams P, et al. Tolterodine treatment improves storage symptoms suggestive of overactive bladder in men treated with alpha-blockers. Eur Urol 2009 Sep;56(3):534-41. PMID: 19070418.
- MacDiarmid SA, Peters KM, Chen A, et al. Efficacy and safety of extended-release oxybutynin in combination with tamsulosin for treatment of lower urinary tract symptoms in men: randomized, double- blind, placebo-controlled study. Mayo Clin Proc 2008 Sep;83(9):1002-10. PMID: 18775200.
- Kaplan SA, Roehrborn CG, Rovner ES, et al. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial.[Erratum appears in JAMA. 2007 Mar 21:297(11):1195], [Erratum appears in JAMA. 2007 Oct 24;298(16):1864]. Jama 2006 Nov 15;296(19):2319-28. PMID: 17105794.
- Kaplan SA, McCammon K, Fincher R, et al. Safety and tolerability of solifenacin add-on therapy to alpha- blocker treated men with residual urgency and frequency. J Urol 2013 Jan;189(1 Suppl):S129-34. PMID: 23234618.
- Chapple CR, Herschorn S, Abrams P, et al. Efficacy and safety of tolterodine extended-release in men with overactive bladder symptoms treated with an alpha- blocker: effect of baseline prostate-specific antigen concentration. BJU Int 2010 Nov;106(9):1332-8. PMID: 20497416.
- Roehrborn CG, Kaplan SA, Jones JS, et al. Tolterodine extended release with or without tamsulosin in men with lower urinary tract symptoms including overactive bladder symptoms: effects of prostate size. Eur Urol 2009 Feb;55(2):472-9. PMID: 18583022.
- Roehrborn CG, Kaplan SA, Kraus SR, et al. Effects of serum PSA on efficacy of tolterodine extended release with or without tamsulosin in men with LUTS, including OAB. Urology 2008 Nov;72(5):1061-7; discussion 7. PMID: 18817961.
- Kaplan SA, Roehrborn CG, Chancellor M, et al. Extended-release tolterodine with or without tamsulosin in men with lower urinary tract symptoms and overactive bladder: effects on urinary symptoms assessed by the International Prostate Symptom Score. BJU Int 2008 Nov;102(9):1133-9. PMID: 18510659.
- Ichihara K, Masumori N, Fukuta F, et al. A randomized controlled study of the efficacy of tamsulosin monotherapy and its combination with mirabegron for overactive bladder induced by benign prostatic obstruction. J Urol 2014 01 Mar;193(3):921- 6. PMID: -25254938.
- Nitti VW, Rosenberg S, Mitcheson DH, et al. Urodynamics and safety of the beta3-adrenoceptor agonist mirabegron in males with lower urinary tract symptoms and bladder outlet obstruction. J Urol 2013 Oct;190(4):1320-7. PMID: 23727415.
- Roehrborn CG, Casabe A, Glina S, et al. Treatment satisfaction and clinically meaningful symptom improvement in men with lower urinary tract symptoms and prostatic enlargement secondary to benign prostatic hyperplasia: Secondary results from a 6-month, randomized, double-blind study comparing finasteride plus tadalafil with finasteride plus placebo. Int J Urol 2015 Jun;22(6):582-7. PMID: 25827166.
- Oelke M, Shinghal R, Sontag A, et al. Time to onset of clinically meaningful improvement with tadalafil 5 mg once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: analysis of data pooled from 4 pivotal, double-blind, placebo controlled studies. J Urol 2015 May;193(5):1581-9. PMID: 25437533.
- Nishizawa O, Yoshida M, Takeda M, et al. Tadalafil 5 mg once daily for the treatment of Asian men with lower urinary tract symptoms secondary to benign prostatic hyperplasia: analyses of data pooled from three randomized, double-blind, placebo-controlled studies. Int J Urol 2015 Apr;22(4):378-84. PMID: 25711404.
- Nickel JC, Brock GB, Herschorn S, et al. Proportion of tadalafil-treated patients with clinically meaningful improvement in lower urinary tract symptoms associated with benign prostatic hyperplasia - integrated data from 1,499 study participants. BJU Int 2015 May;115(5):815-21. PMID: 25195970.
- Takeda M, Yokoyama O, Lee SW, et al. Tadalafil 5 mg once-daily therapy for men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: results from a randomized, double-blind, placebo-controlled trial carried out in Japan and Korea. Int J Urol 2014 Jul;21(7):670-5. PMID: 24571205.
- Singh DV, Mete UK, Mandal AK, et al. A comparative randomized prospective study to evaluate efficacy and safety of combination of tamsulosin and tadalafil vs. tamsulosin or tadalafil alone in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. Journal of Sexual Medicine 2014 Jan;11(1):187-96. PMID: 24165272.
- Kumar S, Kondareddy C, Ganesamoni R, et al. Randomized controlled trial to assess the efficacy of the combination therapy of alfuzosin and tadalafil in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. LUTS: Lower Urinary Tract Symptoms 2014 January;6(1):35-40. PMID: - 26663498.
- Casabe A, Roehrborn CG, Da Pozzo LF, et al. Efficacy and safety of the coadministration of tadalafil once daily with finasteride for 6 months in men with lower urinary tract symptoms and prostatic enlargement secondary to benign prostatic hyperplasia. J Urol 2014 Mar;191(3):727-33. PMID: 24096118.
- Yokoyama O, Yoshida M, Kim SC, et al. Tadalafil once daily for lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a randomized placebo- and tamsulosin-controlled 12- week study in Asian men. Int J Urol 2013 Feb;20(2):193-201. PMID: 22958078.
- Regadas RP, Reges R, Cerqueira JBG, et al. Urodynamic effects of the combination of tamsulosin and daily tadalafil in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia: A randomized, placebo-controlled clinical trial. International Urology and Nephrology 2013 February;45(1):39-43. PMID: -23108604.
- Abolyosr A, Elsagheer GA, Abdel-Kader MS, et al. Evaluation of the effect of sildenafil and/or doxazosin on Benign prostatic hyperplasia-related lower urinary tract symptoms and erectile dysfunction. Urology Annals 2013 October-December;5(4):237-40. PMID: - 24311901.
- Takeda M, Nishizawa O, Imaoka T, et al. Tadalafil for the Treatment of Lower Urinary Tract Symptoms in Japanese Men with Benign Prostatic Hyperplasia: Results from a 12-week Placebo-controlled Dose- finding Study with a 42-week Open-label Extension. LUTS: Lower Urinary Tract Symptoms 2012 September;4(3):110-9. PMID: -26676616.
- Ozturk MI, Kalkan S, Koca O, et al. Efficacy of alfuzosin and sildenafil combination in male patients with lower urinary tract symptoms. Andrologia 2012 May;44 Suppl 1:791-5. PMID: 22211956.
- Oelke M, Giuliano F, Mirone V, et al. Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo-controlled clinical trial. Eur Urol 2012 May;61(5):917-25. PMID: 22297243.
- Madani AH, Afsharimoghaddam A, Roushani A, et al. Evaluation of Tadalafil effect on lower urinary tract symptoms of benign prostatic hyperplasia in patients treated with standard medication. Int Braz J Urol 2012 Jan-Feb;38(1):33-9. PMID: 22397784.
- Goldfischer E, Kowalczyk JJ, Clark WR, et al. Hemodynamic effects of once-daily tadalafil in men with signs and symptoms of benign prostatic hyperplasia on concomitant alpha1-adrenergic antagonist therapy: results of a multicenter randomized, double-blind, placebo-controlled trial. Urology 2012 Apr;79(4):875-82. PMID: 22341603.
- Gacci M, Vittori G, Tosi N, et al. A randomized, placebo-controlled study to assess safety and efficacy of vardenafil 10 mg and tamsulosin 0.4 mg vs. tamsulosin 0.4 mg alone in the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Journal of Sexual Medicine 2012 Jun;9(6):1624-33. PMID: 22510238.
- Egerdie RB, Auerbach S, Roehrborn CG, et al. Tadalafil 2.5 or 5 mg administered once daily for 12 weeks in men with both erectile dysfunction and signs and symptoms of benign prostatic hyperplasia: results of a randomized, placebo-controlled, double-blind study. Journal of Sexual Medicine 2012 Jan;9(1):271- 81. PMID: 21981682.
- Porst H, Kim ED, Casabe AR, et al. Efficacy and safety of tadalafil once daily in the treatment of men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: results of an international randomized, double-blind, placebo-controlled trial. Eur Urol 2011 Nov;60(5):1105-13. PMID: 21871706.
- Kim SC, Park JK, Kim SW, et al. Tadalafil Administered Once Daily for Treatment of Lower Urinary Tract Symptoms in Korean men with Benign Prostatic Hyperplasia: Results from a Placebo- Controlled Pilot Study Using Tamsulosin as an Active Control. LUTS: Lower Urinary Tract Symptoms 2011 September;3(2):86-93. PMID: 26676392.
- Tuncel A, Nalcacioglu V, Ener K, et al. Sildenafil citrate and tamsulosin combination is not superior to monotherapy in treating lower urinary tract symptoms and erectile dysfunction. World J Urol 2010 January;28(1):17-22. PMID: 19855976.
- Dmochowski R, Roehrborn C, Klise S, et al. Urodynamic effects of once daily tadalafil in men with lower urinary tract symptoms secondary to clinical benign prostatic hyperplasia: a randomized, placebo controlled 12-week clinical trial. J Urol 2010 Mar;183(3):1092-7. PMID: 20092847.
- Liguori G, Trombetta C, De Giorgi G, et al. Efficacy and safety of combined oral therapy with tadalafil and alfuzosin: an integrated approach to the management of patients with lower urinary tract symptoms and erectile dysfunction. Preliminary report. Journal of Sexual Medicine 2009 Feb;6(2):544-52. PMID: 19138360.
- Stief CG, Porst H, Neuser D, et al. A randomised, placebo-controlled study to assess the efficacy of twice-daily vardenafil in the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur Urol 2008 Jun;53(6):1236-44. PMID: 18281145.
- Roehrborn CG, McVary KT, Elion-Mboussa A, et al. Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a dose finding study. J Urol 2008 Oct;180(4):1228-34. PMID: 18722631.
- McVary KT, Roehrborn CG, Kaminetsky JC, et al. Tadalafil relieves lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol 2007 Apr;177(4):1401-7. PMID: 17382741.
- McVary KT, Monnig W, Camps Jr JL, et al. Sildenafil Citrate Improves Erectile Function and Urinary Symptoms in Men With Erectile Dysfunction and Lower Urinary Tract Symptoms Associated With Benign Prostatic Hyperplasia: A Randomized, Double-Blind Trial. J Urol 2007 March;177(3):1071- 7. PMID: -17296414.
- Kaplan SA, Gonzalez RR, Te AE. Combination of Alfuzosin and Sildenafil is Superior to Monotherapy in Treating Lower Urinary Tract Symptoms and Erectile Dysfunction. Eur Urol 2007 June;51(6):1717- 23. PMID: 17258855.
- Roehrborn CG, Chapple C, Oelke M, et al. Effects of tadalafil once daily on maximum urinary flow rate in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. J Urol 2014;191(4):1045-50. PMID: 24445278.
- Oelke M, Weiss JP, Mamoulakis C, et al. Effects of tadalafil on nighttime voiding (nocturia) in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a post hoc analysis of pooled data from four randomized, placebo-controlled clinical studies. World J Urol 2014 Oct;32(5):1127-32. PMID: 24504761.
- Oelke M, Giuliano F, Baygani SK, et al. Treatment satisfaction with tadalafil or tamsulosin vs placebo in men with lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH): results from a randomised, placebo-controlled study. BJU Int 2014 Oct;114(4):568-75. PMID: 24612148.
- Lee SW, Paick JS, Park HJ, et al. The Efficacy and Safety of Tadalafil 5 mg Once Daily in Korean Men with Lower Urinary Tract Symptoms Suggestive of Benign Prostatic Hyperplasia: An Integrated Analysis. World j 2014 Apr;32(1):28-35. PMID: 24872949.
- Brock GB, McVary KT, Roehrborn CG, et al. Direct effects of tadalafil on lower urinary tract symptoms versus indirect effects mediated through erectile dysfunction symptom improvement: Integrated data analyses from 4 placebo controlled clinical studies. J Urol 2014;191(2):405-11. PMID: 24096120.
- Porst H, Roehrborn CG, Secrest RJ, et al. Effects of tadalafil on lower urinary tract symptoms secondary to benign prostatic hyperplasia and on erectile dysfunction in sexually active men with both conditions: analyses of pooled data from four randomized, placebo-controlled tadalafil clinical studies. Journal of Sexual Medicine 2013 Aug;10(8):2044-52. PMID: 23782459.
- Porst H, Oelke M, Goldfischer ER, et al. Efficacy and safety of tadalafil 5 mg once daily for lower urinary tract symptoms suggestive of benign prostatic hyperplasia: subgroup analyses of pooled data from 4 multinational, randomized, placebo-controlled clinical studies.[Erratum appears in Urology. 2014 Mar;83(3):684]. Urology 2013 Sep;82(3):667-73. PMID: 23876588.
- Dmochowski R, Roehrborn C, Klise S, et al. Urodynamic effects of once daily tadalafil in men with lower urinary tract symptoms secondary to clinical benign prostatic hyperplasia: a randomized, placebo controlled 12-week clinical trial. J Urol 2013 Jan;189(1 Suppl):S135-40. PMID: 23234619.
- Brock G, Broderick G, Roehrborn CG, et al. Tadalafil once daily in the treatment of lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH) in men without erectile dysfunction. BJU Int 2013 Nov;112(7):990-7. PMID: 23937669.
- Broderick GA, Brock GB, Roehrborn CG, et al. Effects of tadalafil on lower urinary tract symptoms secondary to benign prostatic hyperplasia in men with or without erectile dysfunction. Urology 2010 Jun;75(6):1452-8. PMID: 20163842.
- McVary KT, Siegel RL, Carlsson M. Sildenafil Citrate Improves Erectile Function and Lower Urinary Tract Symptoms Independent of Baseline Body Mass Index or LUTS Severity. Urology 2008 September;72(3):575-9. PMID: 18597830.
- Drake MJ, Chapple C, Sokol R, et al. Long-term Safety and Efficacy of Single-tablet Combinations of Solifenacin and Tamsulosin Oral Controlled Absorption System in Men with Storage and Voiding Lower Urinary Tract Symptoms: Results from the NEPTUNE Study and NEPTUNE II Open-label Extension. Eur Urol 2015 Feb;67(2):262-70. PMID: 25070148.
- Yoshimura K, Kadoyama K, Sakaeda T, et al. A survey of the FAERS database concerning the adverse event profiles of alpha1-adrenoreceptor blockers for lower urinary tract symptoms. Int J Med Sci 2013;10(7):864-9. PMID: 23781132.
- Donatucci CF, Brock GB, Goldfischer ER, et al. Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a 1-year, open-label extension study. BJU Int 2011 Apr;107(7):1110-6. PMID: 21244606.
- Marks LS, Gittelman MC, Hill LA, et al. Silodosin in the treatment of the signs and symptoms of benign prostatic hyperplasia: a 9-month, open-label extension study. Urology 2009 Dec;74(6):1318-22. PMID: 19815265.
- Kloner RA. Pharmacology and drug interaction effects of the phosphodiesterase 5 inhibitors: focus on alpha- blocker interactions. Am J Cardiol 2005 Dec 26;96(12B):42M-6M. PMID: 16387566.
- Lau J, Terrin N, Fu R, eds. Expanded Guidance on Selected Quantitative Synthesis Topics. Methods Research Report. (Prepared by the Tufts Evidence- based Practice Center under Contract No. 290-2007- 10055-I.) AHRQ Publication No. 13-EHC024-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- Blanker MH, van Deventer KR, Bijl D. Measuring symptomatic relief in men with lower urinary tract symptoms. BMJ. 2014;349:g6664. PMID: 25380756. Leggi altro...
L'informazione presente nel sito deve servire a migliorare, e non a sostituire, il rapporto medico-paziente. In caso di disturbi e/o malattie rivolgiti al tuo medico di base o ad uno specialista.
Cerca i migliori specialisti che si occupano di Ipertrofia prostatica benignaRevisione Scientifica

Hai bisogno di un Dottore per Ipertrofia prostatica benigna?
Trova il Medico più adatto alle tue esigenze.
Articoli correlati
Hai bisogno di un Dottore per Ipertrofia prostatica benigna?